Most women with early-stage breast cancer have a higher chance of recovery. Each woman’s breast cancer has unique molecular characteristics which may reflect the tumor aggressiveness and the chance of cancer recurrence. Some of these women (~25%) may still experience a cancer recurrence/relapse after surgery. These recurrences often happen within 5 years of initial diagnosis. Hormonal therapy and chemotherapy are required to lower the risk of cancer recurrence after surgery. However, chemotherapy results in side effects and the absolute benefits may be low for patients with low chances of cancer recurrence — It is generally recommended for patients with less than 10% of distant recurrences to consider withholding chemotherapy. Prosigna is a new test which can reliably identify a patient’s 10-year risk of distant recurrence and may inform on whether chemotherapy is required for post-surgery.
Prosigna
What is Prosigna?
- A Breast Cancer Prognostic Gene Signature Assay based on the characterisation of 50 genes relevant to breast cancer biology
- It identifies the intrinsic tumor subtype based on the individual tumor biology, to predict the patient’s prognostic risk of recurrence (ROR) score and the probability of cancer recurrence within 10 years
- It has been being approved by major regulatory bodies, such as FDA 510(k) and CE-IVD. It is also endorsed by independent regulatory bodies and professional oncology organisations worldwide
Why choose Prosigna?
- Validated on >5000 samples in the combined ABCSG-8, TransATAC and DGBC studies
- The test is FDA 510(k) cleared and CE-marked
- Recognized and listed in multiple international guidelines including the latest NICE recommendation*, ASCO, NCCN, and ESMO
- Prosigna is being run in a CAP- accredited lab in Singapore
- Prosigna is much more cost-effective compared with other genomic profiling products
*Since December 2018, the Prosigna Assay is also recommended by NICE (UK) for the guidance of chemotherapy treatment decisions, for early stage breast cancer patients in the United Kingdom. NICE (UK) is broadly regarded as a world leader in the assessment of clinical and cost effectiveness. This endorsement affirms the clinical value of the Prosigna assay to inform treatment decisions.
Who should take Prosigna?
- Post-menopausal women with a diagnosis of early-stage breast cancer undergoing mastectomy or breast-conserving surgery
- Hormone receptor positive (ER and/or PR)
- Node-negative and node positive (1-3 nodes)
- Tumor size <5cm
- Post-surgical early stage breast cancer (stage I, II, IIIA breast cancer)
What is the process?
Speak with your physician to understand whether Prosigna is a suitable test for you. The test does not require additional surgery or procedure. The test can be performed on the cancer tissue specimen preserved from your original surgery. The results will be ready within 10 working days upon specimen receipt in our laboratory.
What information does Prosigna provide in your report?
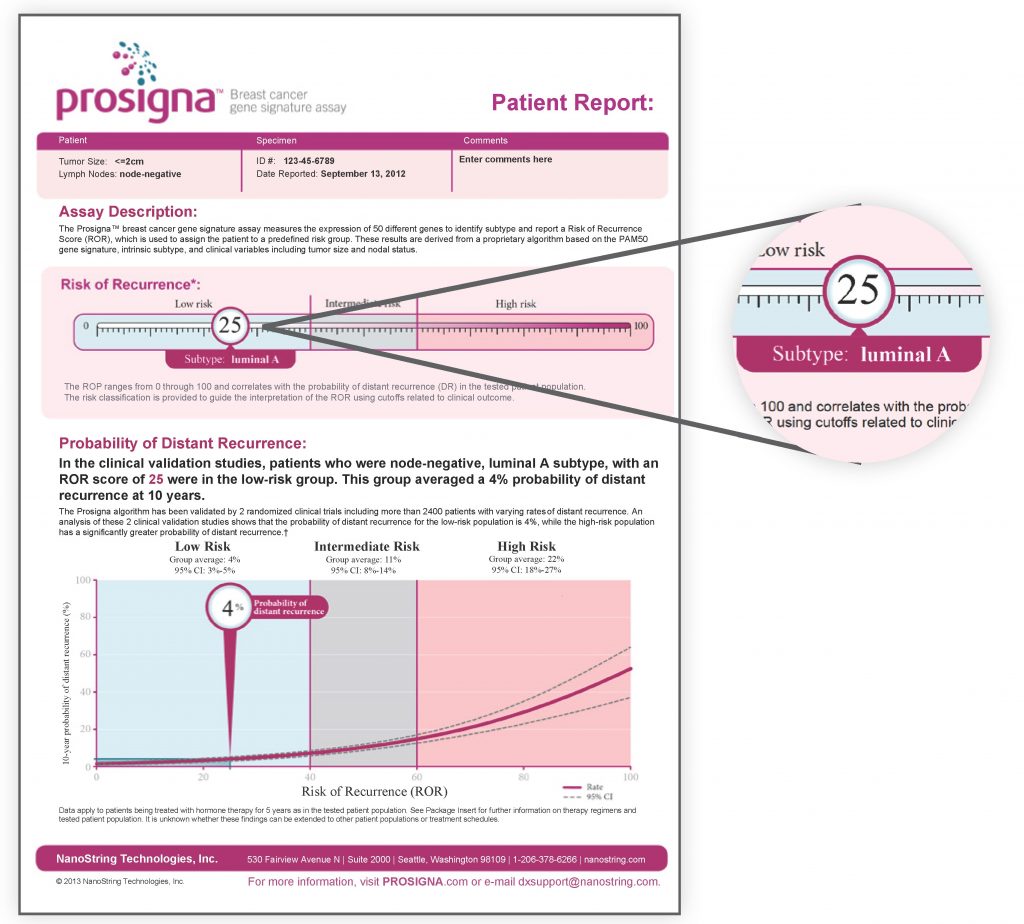
Prosigna provides a rapid, reliable and individualized assessment of risk. It will provide the following information to guide your doctor for a better treatment plan:
- Intrinsic subtype of breast cancer: This identifies your intrinsic tumor subtypes, which may be classified as one of four molecular classes: Luminal A, Luminal B, HER2 enriched or Basal-like. Tumor subtypes provide additional information to your doctor regarding the underlying biology, aggressiveness and likely responses of your cancer to treatment.
- Prosigna score: This is a numerical value on a 0-to-100 scale. A lower number indicates that your cancer is less likely to return. A higher number means there is a higher chance your cancer may return.
- Risk of cancer recurrence: The risk of recurrence is provided as a percentage. This result indicates the likelihood of your cancer will return within the next 10 years, when treated solely with hormonal therapy.
Accreditation
Now included in the International Clinical Practice Guidelines, Prosigna by Nanostring detects early stage breast cancer in patients through a highly accurate, FDA-approved risk assessment test. With the use of the PAM50 gene signature to translate tumour biology into a patient’s individualised prognostic score, Prosigna also identifies the patient’s 10-year risk of distant recurrence and intrinsic molecular subtype.


-
Benefits
- FDA-approved
- Predicts the possibility of a cancer recurrence over the next 10 years
- Enables patients to make an informed decision about cancer treatment after surgery
- Helps healthcare professionals decide if hormone therapy is sufficient and/or if other treatments are required
-
Recommended For
- Post-menopausal women with newly diagnosed, early stage invasive breast cancer treated with endocrine therapy
- Women who are hormone receptor positive (estrogen or progesterone)
- Women who are lymph-node negative or lymph node-positive
-
Process
The test does not require additional surgery and can be performed on cancer tissue specimen preserved from original operations.
Results will be released within 10 business days upon receipt of the tissue specimen.
-
Downloads