NOVOPM™
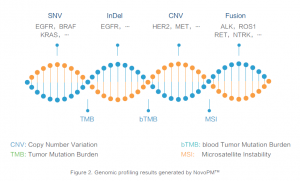
Comprehensive Cancer Genomic Profiling test, NovoPM™ provides clinicians, a tool to study the genetic causes of solid cancer and identify potential targeted therapies available. By analysing over 450 genes for clinically important alteration, which are known to be relevant for the diagnosis and/or treatment of various solid tumours according to National Comprehensive Cancer Network (NCCN) guidelines and medical literature.